The expression of exogenous genes in target cells usually requires the introduction of viral or non-viral vectors containing exogenous DNA fragments. In clinical applications, using the traditional methods that direct introduction of exogenous DNA into the human body have certain safety issues. In contrast, in vitro transcribed(IVT) mRNA has natural advantages in safety due to its non infectious ability, unconformity genome and natural degradation in cells. Therefore, it is also a popular tool for gene therapy and new vaccine research.
 Advantages
Advantages
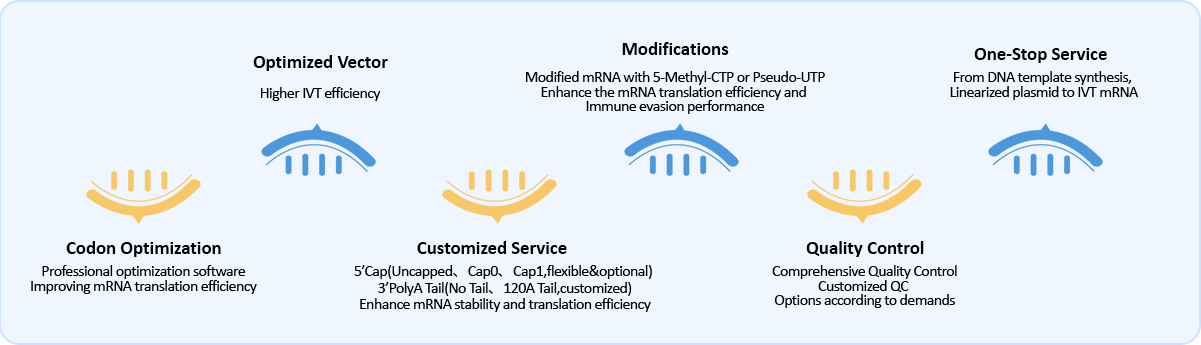
Gene Universal have professional and rich experience technical team for custom IVT mRNA synthesis, now offers one-stop service from Gene synthesis, Plasmid Preparation, linearized plasmid to IVT mRNA synthesis. The length range of IVT mRNA is from 100nt to 5000nt, it can be optimized and customized for different downstream applications, such as template optimization, coding region codon optimization, nucleoside modification, and post transcriptional modification, etc. IVT mRNA can be applied in research fields such as cell transfection, embryo injection, bio-pharmaceuticals, and vaccine development, etc.
 Service Features
Service Features
-
Free codon optimization, improving mRNA translation efficiency
-
Optimized vector, have higher IVT efficiency
-
Custom cap and tail: 5’ cap(Uncapped、Cap0、Cap1) and 3’ PolyA Tail(No Tail、120A Tail, customized), to enhance mRNA stability and translation efficiency
-
Modifications like 5-Methyl-CTP、Pseudo-UTP, which can enhance the mRNA translation efficiency and immune evasion performance
-
Comprehensive quality control plan, customized QC projects according to needs
-
One-stop service from DNA template synthesis to IVT mRNA
-
Rapid delivery – Industry-leading turnaround time
 Service Options
Service Options
| Services Step | Service Content | Turnaround Time | Price |
|---|---|---|---|
| 1. Gene synthesis, Plasmid Preparation | Gene synthesis、Plasmid construction | 2-3 weeks | Inquiry |
| Plasmid preparation | |||
| 2. IVT mRNA Synthesis | Linearized plasmid | 3-4weeks | |
| IVT, add 5'cap and 3'PolyA tail, modification | |||
| 3. Quality Control | The default QC+extra QC item(Customized) | Inquiry |
 QC Item
QC Item
| QC | Item | Test Method | Specification |
|---|---|---|---|
| Default | mRNA Length | Agarose gel electrophoresis | Expected size band detected |
| mRNA Sequence | Sanger sequencing | Correct DNA template | |
| Concentration | Nano drop or Qubit | 1μg/μl(Default) | |
| Appearance | Visual inspect | Clear, no visible particles | |
| A260/280 | Nano drop | 2.0-2.2 | |
| Purity | HPLC | ≥90% | |
| Extra(optional)* | Residual DNA template | qPCR | ≤ 10pg/ug |
| Endotoxin level | Gel-TAL | ≤0.005 EU/μg | |
| Residual Protein | ELISA or Qubit | ≤ 1% | |
| Bioburden | Plate | 48h sterile spots |
* Need Charge extra fee for extra QC items.
 Standard Deliverables
Standard Deliverables
-

DNA Sequence verified via
Sanger sequencing
-

Purity tested by
gel -electrophoresis
-

Lyophilized mRNA
-

QC report
 Case presentation
Case presentation
Agarose gel electrophoresis of mRNA
-
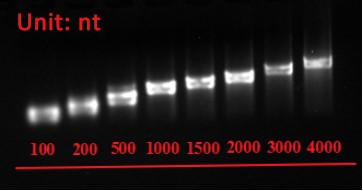
Figure 1. Different length of mRNA
-
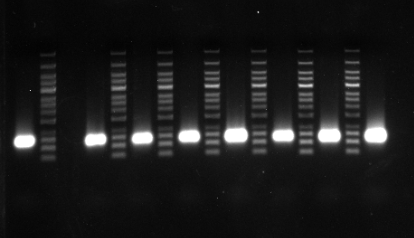
Figure 2. 200 nt mRNA
mRNA Purity Test
By use the Linearized plasmid or PCR product as templates, the mRNA Purity>90%
-
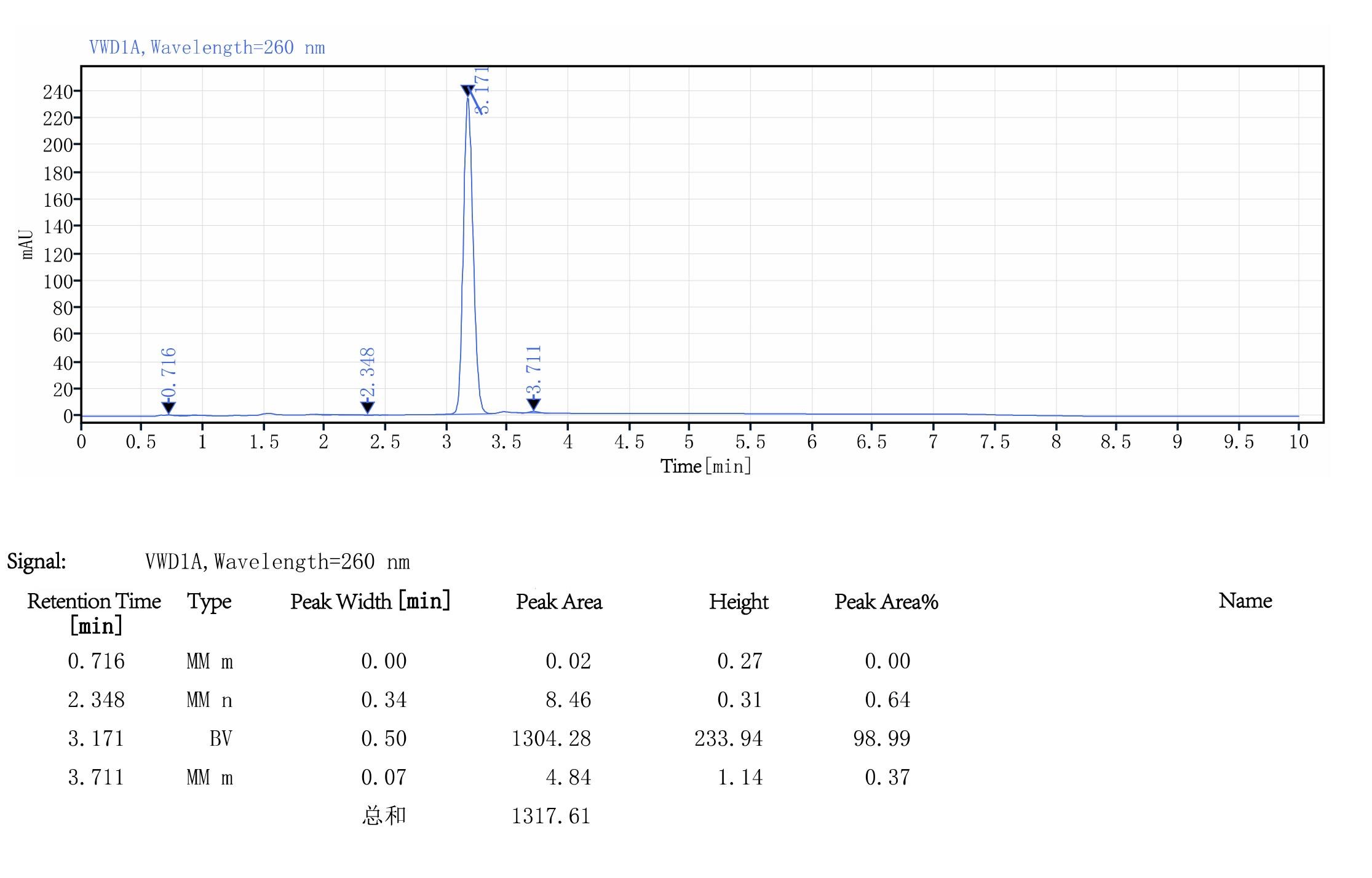
Figure 3. Linearized plasmid template:Purity Test of 100 nt mRNA
-
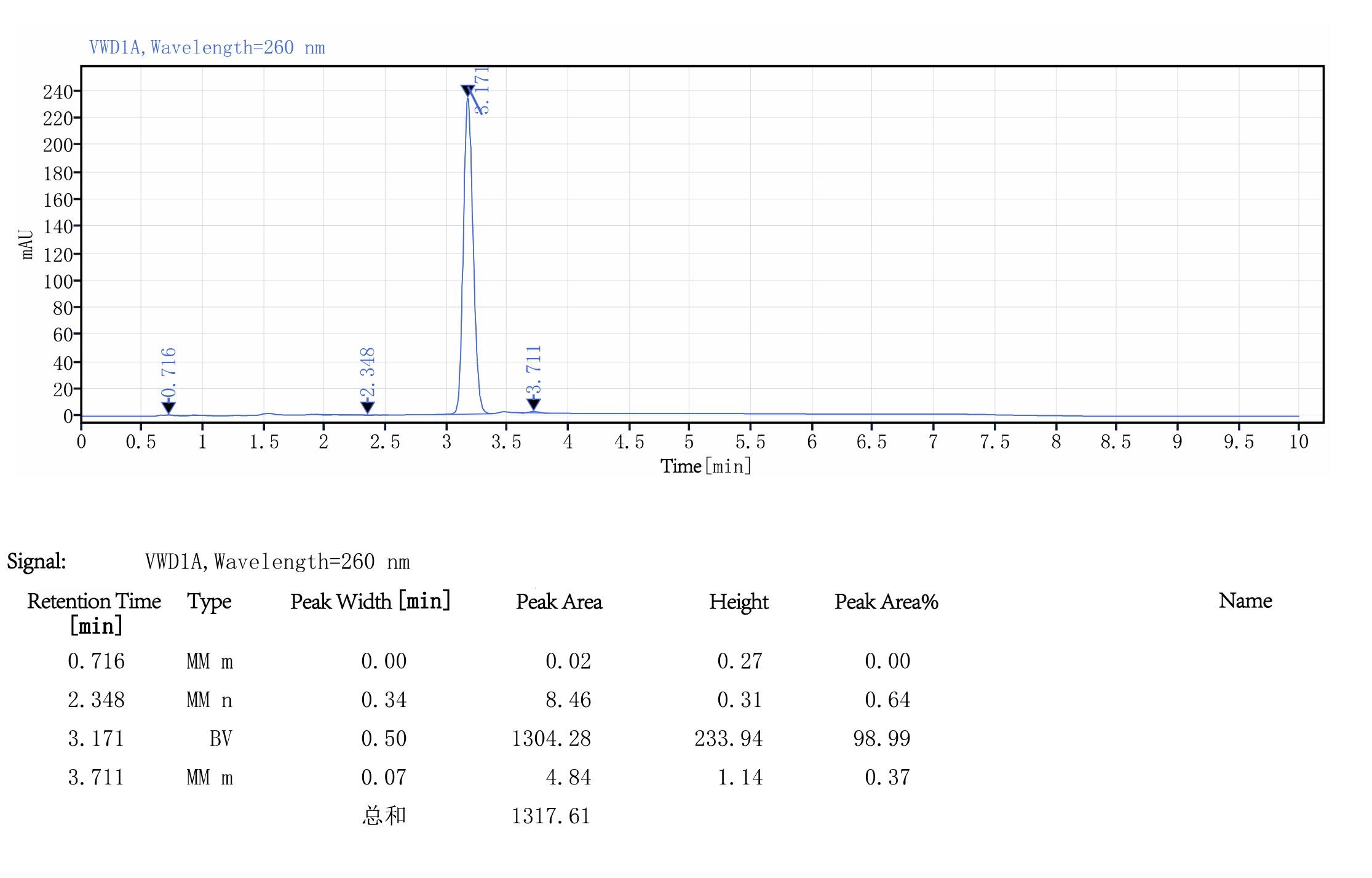
Figure 4. PCR product template:Purity Test of 100 nt mRNA
 How to Order?
How to Order?